Date set for DXB clinical trial result
Today our 2021 Biotech Pick of the Year Dimerix (ASX:DXB) announced that the 72nd patient has now been randomised for the Phase III clinical study.
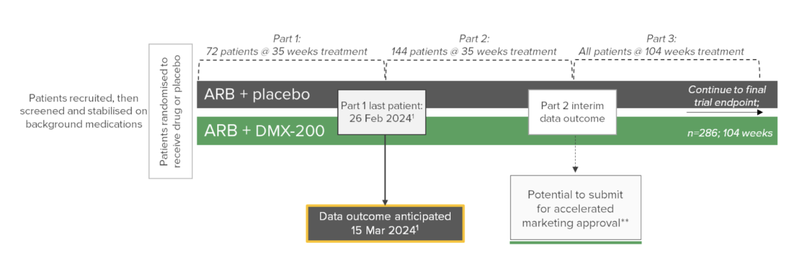
DXB is currently undertaking a Phase III clinical trial for a rare kidney disease called FSGS.
FSGS is an “orphan disease” which means that it is eligible for accelerated marketing approvals and orphan drug pricing.
The significance of this news today is that the company now has a clear timeline on when interim data outcome is due - March 15, 2024.
Like with a lot of early stage biotech companies “delay risk” has manifested for DXB.
The company announced the 72nd patient had been recruited in December of 2022, and what was meant to be a six week “stabilisation” period has unfortunately been stretched out to six months.
That said, the last patient has now been put on the clinical trial pathway.
This is a key milestone that we have been waiting for and gives us a timeline for the interim analysis results.
Basically the “interim analysis” involves an independent committee (a Data Safety Monitoring Board) that has access to data on the trial saying, “yes keep going, DXB’s treatment is working and safe” or a “no stop, it isn’t working or isn’t safe”.
The Data Safety Monitoring Board has a meeting scheduled for March 15, 2024 to assess DXB’s clinical trial progress and a large catalyst for the company.
We think that a positive “yes keep going” result will significantly de-risk the clinical trial and put DXB at the forefront of any cashed up big pharma company looking to boost their presence in the renal (kidney) space.
We deep dive into what a potential could look like for DXB in our latest note: US$3.5BN kidney deal puts DXB’s current valuation in perspective.
Also of significance is that DXB has been recruiting through the process and now has 133 patients recruited (72 on randomised treatment and 61 in the stabilisation period).
This is just 11 shy of the 144 needed for the second interim data outcome.
Unlike the first interim analysis results, which just measures whether there is enough evidence to suggest that the clinical trial keeps going, the second interim analysis results will identify if DXB is eligible for marketing approval.
What’s next for DXB?
✅ 72nd patient commenced (randomised) treatment
🔄 Completion of recruitment (144 patients)
🔄 The big catalyst: interim analysis results (Part 1 data outcome)
- Bull Case: Enough evidence to continue the trial
- Bear Case: Trial paused or stopped






